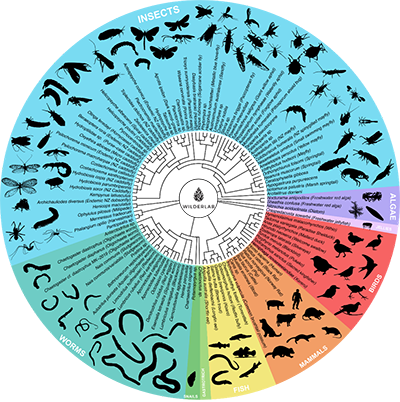
“Think of bar codes on a product at a supermarket; every product that goes through the checkout has a bar code, as do the different organisms that are being detected by this technology.”
– Bruno David, Water Scientist
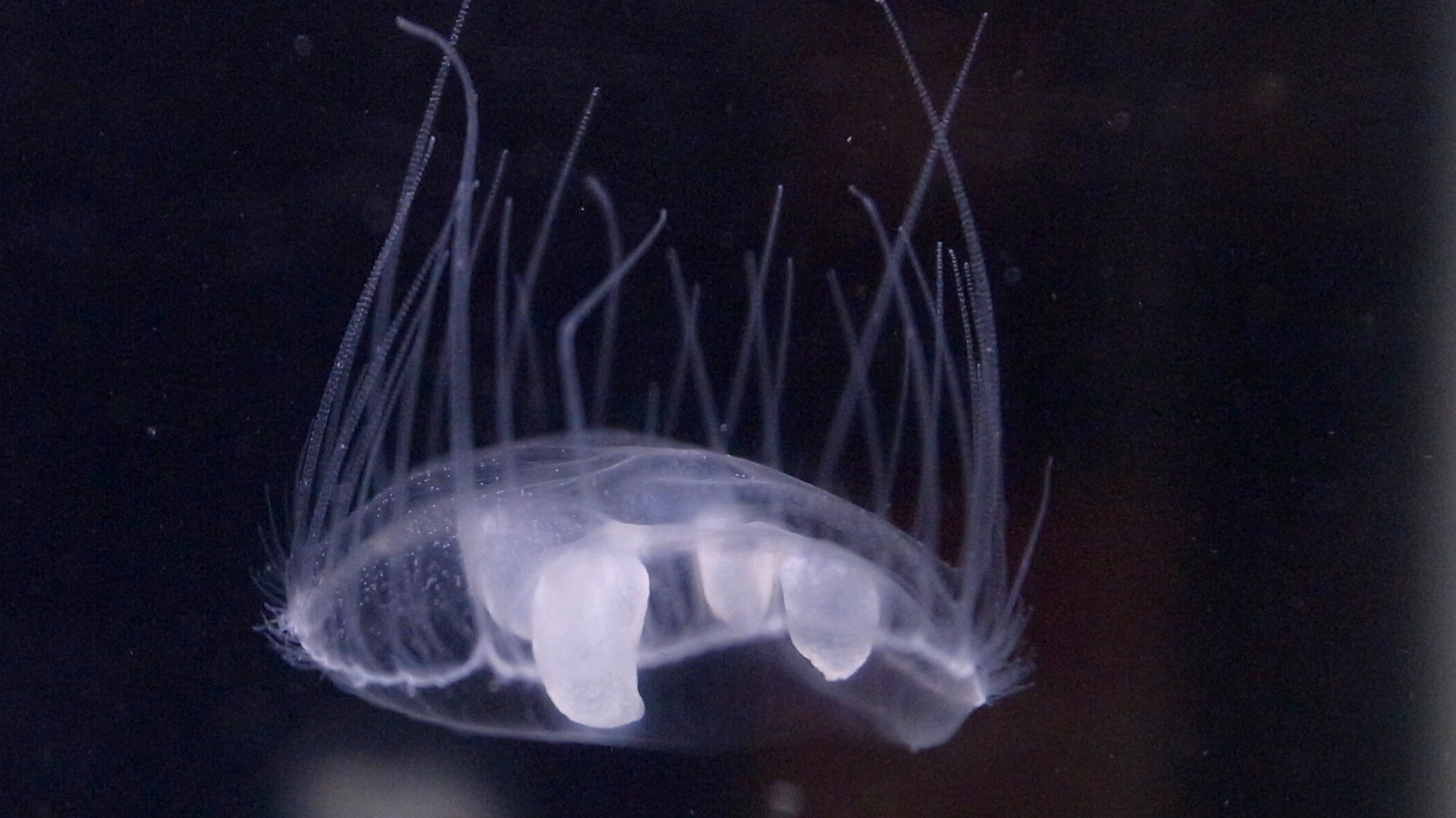
Water scientist Bruno David has been working in fresh water in New Zealand for many years, but he had no idea that an alien predator freshwater jellyfish was widespread in rivers across the country.
“It came as quite a surprise to me,” says Bruno, who was among many freshwater scientists to become enlightened about this fact thanks to the adoption of eDNA monitoring in New Zealand.
“We knew we had this little Asian invading jellyfish in lakes, but it came as quite a surprise to us that they are completely widespread across the entire country in rivers.”
 eDNA monitoring is a rapidly evolving, cost-effective tool with substantial potential to improve knowledge of the management of aquatic habitats.
eDNA monitoring is a rapidly evolving, cost-effective tool with substantial potential to improve knowledge of the management of aquatic habitats.
Environmental DNA, or eDNA, refers to all the fragments of genetic material that are left behind as living things pass through water or soil. By collecting DNA shed in the environment and sequencing it, we can get a picture of the plants and animals in a local area.
“I’m very sceptical, as I think most scientists are, and I was very sceptical of this type of technology to begin with,” says Bruno. “But I had seen it used overseas quite a bit, so naturally I started to test it in places where we had pre-existing information, places where we have 12-13 years of data, and it was pretty much bang on at identifying the species we knew were present in those places. It’s highly sensitive technology, and it’s relatively quick and efficient, not resource intensive.”
Bruno says eDNA is mostly collected from water, particularly rivers, “because they act like a conveyor belt of DNA” but there are many applications for the technology in other environment types.
“There is always eDNA coming down a river. It’s delivered to you rather than having to go find it, and it doesn’t just come from the things living in the river but also things interacting with riparian areas outside of it. So, if a bird sitting in canopy defecates in the water, its DNA will end up on that conveyor belt.
“We can use eDNA monitoring for identifying biosecurity threats, such as koi in waterways, and threatened species detection work or looking at things like stream rehabilitation. Let’s say someone does a planting project for rehabilitation, you can take samples beforehand and after the fact, and into the future. By doing that, you might be able to look at how long it takes before certain species starts to arrive at the rehabilitation site.”
eDNA is collected using special kits that filter water and trap biological material. The DNA can then be extracted from the material captured on the filter in a lab.
“Think of bar codes on a product at a supermarket; every product that goes through the checkout has a bar code, as do the different organisms that are being detected by this technology. Except with this technology, we can run lots of different barcodes at the same time instead of scanning them one by one. They are run across a global database which has all the sequence information for animals around the world.”
Bruno says Wilderlab, which processes the council’s eDNA samples and is exclusively an eDNA testing laboratory, has an interactive map on its website which the public can use to explore the collection of publicly available eDNA data which has been made available by its clients. eDNA field sampling kits can also be ordered online from as little as $15.
“This is one of the few tools I’ve come across where citizen scientists can actually collect some pretty robust data,” says Bruno.
In fact, the Environmental Protection Authority’s Open Waters Aotearoa project encourages community groups, hapū and schools to explore and discover their local waterways by offering eDNA testing kits to those who havent used them before.
“eDNA monitoring just opens up a whole new world and the technology and worldwide database is just improving all the time,” says Bruno.
“Of course, as DNA sheds all the time from both live an dead organism and because the methods is highly sensitive to detection, caution needs to be exercised when taking samples. So, if you just had a yellow-fin tuna sandwich for lunch, and you don’t take care and use gloves when sampling, you’re going to pick up yellowfin tuna in the sample.
“This also shows it’s important for people to recognise that the presence of something doesn’t always mean it could be there. For example, we recently found very high numbers of snapper and kahawai DNA in a freshwater system down in the Awakino and discovered a whole bunch of fish frames in the stream. They were roughly a kilometre or so above the site we were sampling.

To ask for help or report a problem, contact us
Tell us how we can improve the information on this page. (optional)